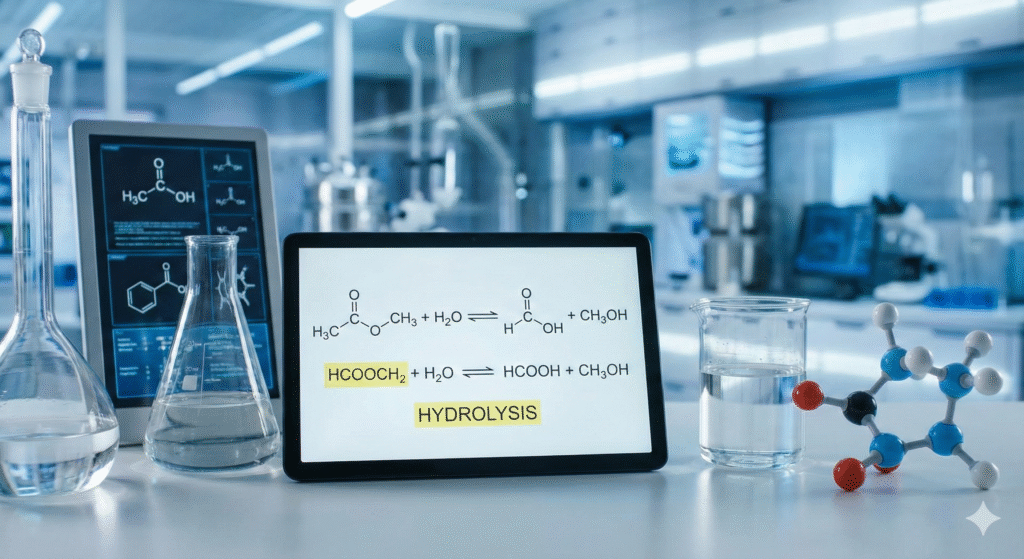
In the fast-evolving landscape of chemical engineering and modern technology, certain molecular reactions serve as the hidden engines of industry. One such fundamental interaction involves esters and water, often searched by students and professionals alike as hcooch ch2 h2o. This string of characters represents the components involved in the hydrolysis of methyl formate—a process that is currently revolutionizing green chemistry and industrial manufacturing.
Why is this relevant to the modern tech world? Whether you are a chemical engineering student, a lab technician, or simply a technology enthusiast, understanding how these specific molecules interact unlocks secrets about fuel cell development, biodegradable plastics, and efficient energy storage. This guide breaks down the science, the technology, and the real-world applications of this unique chemical system, offering a clear window into the molecular tech powering our future.
Unpacking the hcooch ch2 h2o Chemical System
To the untrained eye, hcooch ch2 h2o might look like a random code, but in professional chemistry, it acts as a shorthand for a specific set of molecular interactions. It refers to Methyl Formate (HCOOCH3)—which contains a methyl group often noted in shorthand involving CH2 or CH3 structures—reacting with Water (H2O).
When these components mix under precise technological conditions, they undergo hydrolysis. This means the water molecule acts to split the ester bond, creating new, highly useful compounds. This reaction is the backbone of many “green” chemical processes used in factories today.
The Role of Methyl Formate in Modern Industry
Methyl formate is a powerhouse in the tech and manufacturing sectors. It is widely used as a blowing agent for the foam insulation found in smart appliances and as a solvent in high-tech cleaning processes. The hcooch ch2 h2o reaction is the primary commercial method for generating high-purity formic acid without creating the salty waste products associated with older methods.
Tech industries favor this chemical because of its volatility and efficacy. It helps create the intricate internal components of electronics and the durable finishes on electric vehicles. Without this specific molecule, modern manufacturing would be significantly slower and more expensive.
Breaking Down the Molecular Components
To truly grasp the hcooch ch2 h2o system, one must look at the atomic structure.
- HCOOCH3: This is the ester, featuring a carbonyl group (C=O) and an ether link.
- H2O: The universal solvent, water, which acts as the catalyst for change.
- CH2/CH3: These represent the carbon chains attached to the structure, defining its organic nature.
In a reactor, the water attacks the carbonyl carbon in a process that resembles a key fitting into a lock. This precision chemistry unlocks energy and materials used in daily life.
Why Hydrolysis Matters in Chemical Engineering
Hydrolysis is the technical term for “splitting with water.” In the context of the hcooch ch2 h2o keyword, it represents a clean, efficient way to break down complex molecules. Traditional methods often relied on harsh inorganic acids that corroded equipment and created hazardous waste.
This specific hydrolysis path is unique because it is “autocatalytic.” This means the product created by the reaction actually speeds up the reaction itself. It is a smart chemical loop that engineers design to save energy and time in large-scale factories.
Industrial Applications of this Reaction
The real-world footprint of hcooch ch2 h2o is massive.
- Leather Tanning: It produces the specific acids needed to soften leather for high-end tech accessories.
- Textiles: It helps dye fabrics evenly for smart clothing.
- Rubber Production: It is used to coagulate rubber latex efficiently.
Beyond these, the tech industry uses products from this reaction to clean semiconductor wafers. It is a hidden hero in the production pipeline of the gadgets we use every day.
The Tech Behind Synthesizing Formic Acid
The primary goal of mixing hcooch ch2 h2o is usually to synthesize Formic Acid. This acid acts as a vital preservative and antibacterial agent. In the past, producing it was a dirty, chemically aggressive job.
Today, using advanced “reactive distillation” columns, engineers can force this reaction to occur with near 90% efficiency. This technology separates the products instantly, preventing them from reverting to their starting materials—a feat of modern engineering.
Safety Protocols in Chemical Handling
Working with the components of hcooch ch2 h2o requires strict adherence to safety protocols. Methyl formate is highly flammable and volatile.
- Ventilation: Laboratories utilize high-tech fume hoods to clear vapors.
- Sensors: Digital gas detectors are employed to spot leaks immediately.
- PPE: Chemical-resistant gloves and goggles are non-negotiable standards.
Safety technology has evolved rapidly, allowing automated systems to handle these volatile liquids without direct human exposure, making the chemical industry safer than ever before.
Environmental Impact of Chemical Solvents
One of the most significant advantages of the hcooch ch2 h2o system is its “green” profile. The byproducts are often fully recyclable. For instance, Methanol produced during the process can be fed right back into the system to create more methyl formate.
This “circular economy” approach drastically reduces waste. Unlike old solvents that depleted the ozone layer, these chemicals break down naturally in the environment, making them a favorite choice for eco-conscious tech companies.
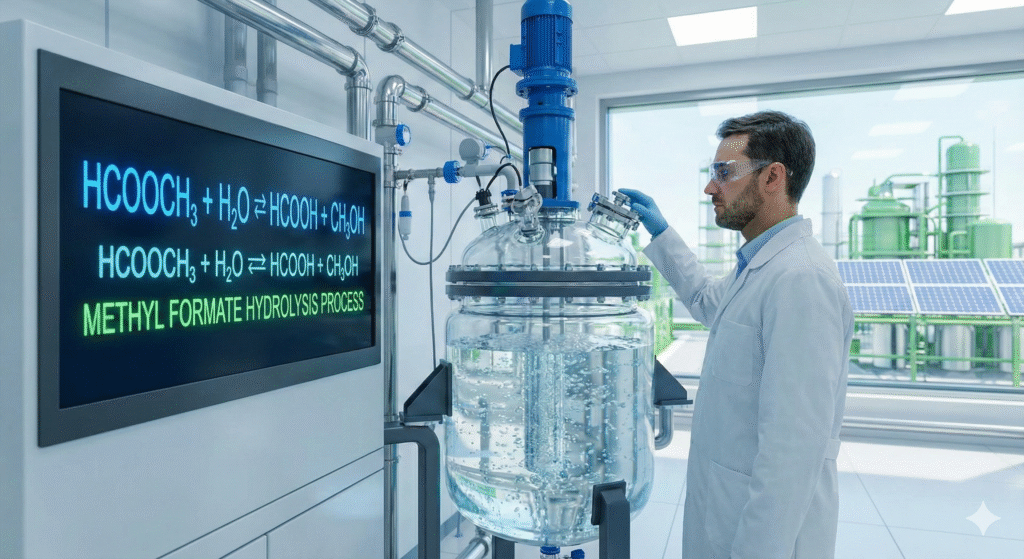
Comparing Hydrolysis Methods
When evaluating efficiency, the hcooch ch2 h2o method stands out against older techniques.
| Feature | hcooch ch2 h2o Hydrolysis | Traditional Acid Hydrolysis |
| Waste Produced | Very Low (Recyclable) | High (Salt Waste) |
| Energy Use | Moderate | High |
| Purity | High (99%+) | Lower (Requires filtering) |
| Equipment Cost | High (Advanced Tech) | Low (Simple Tanks) |
Innovations in Catalytic Converters
To accelerate the hcooch ch2 h2o reaction, scientists utilize solid catalysts. These act like high-tech sponges, holding the molecules in the perfect position to react.
New “macroporous” resins are currently being developed to improve this process. These materials allow the fluid to flow through faster, potentially boosting production rates by 20%. It is a subtle tweak in material science that makes a huge difference in global chemical output.
Solvent Properties and Tech Cleaning
The components involved in hcooch ch2 h2o function as excellent solvents. They are capable of dissolving stubborn greases and oils without damaging sensitive metals.
In the electronics industry, this property is vital. Circuit boards must be perfectly clean before chips are soldered. Using this solvent system ensures that no residue is left behind, preventing short circuits in devices like phones or laptops.
Thermodynamics of the Reaction
Heat plays a critical role in this process. The hcooch ch2 h2o reaction is “equilibrium limited,” meaning it naturally wants to stop halfway before all the material is converted.
Engineers use thermodynamics—the study of heat and energy—to overcome this. By carefully manipulating temperature and pressure, they can push the reaction to completion. This delicate balancing act is managed by sophisticated computer control systems.
Future of Green Chemistry Tech
The future looks incredibly bright for the hcooch ch2 h2o system. Researchers are currently investigating its potential for hydrogen storage. Formic acid, a product of this reaction, can hold hydrogen fuel safely in a liquid state.
This could lead to the development of “liquid batteries” for electric cars. Instead of heavy lithium batteries, vehicles might one day be fueled by a safe liquid derived from this simple chemical reaction.
Shutterstock
Analyzing Reaction Kinetics
Kinetics is the study of speed in chemistry. How fast does hcooch ch2 h2o transform into useful products?
- Temperature: Higher heat generally speeds up the process.
- Concentration: Increasing the water ratio pushes the reaction forward.
Engineers use complex simulation software to model these speeds. They simulate millions of atomic collisions to find the perfect “recipe” for the fastest, most cost-effective production.
Equipment Used in High-Tech Labs
You cannot simply mix these chemicals in a beaker. The hcooch ch2 h2o process utilizes advanced hardware:
- Plug Flow Reactors: Long, tubular vessels where the reaction occurs as liquids flow continuously.
- Spectrometers: Lasers that measure the chemical composition in real-time.
These tools are often connected to the IoT (Internet of Things), allowing scientists to monitor reaction stability remotely from smartphones or tablets.
Digital Simulation of Chemical Reactions
Before a single drop of chemical is physically mixed, the hcooch ch2 h2o system is built in a virtual environment. “Digital Twins” of the factory run simulations 24/7.
This AI-driven tech predicts problems before they physically occur. If a valve is predicted to fail or a mixture ratio is slightly off, the computer warns the engineers. This predictive maintenance saves millions of dollars and ensures worker safety.
Troubleshooting Common Lab Errors
Even professionals encounter issues with the hcooch ch2 h2o process.
- Excess Water: This dilutes the product, making purification difficult and energy-intensive.
- Incorrect Temperature: This can cause the liquid to boil away (vaporize) too quickly before reacting.
The solution is always precise measurement. Modern labs use automated dosing pumps accurate to the microliter, ensuring the perfect stoichiometric ratio every single time.
Economic Value of Formate Derivatives
Finally, the economic impact is substantial. The market for products derived from hcooch ch2 h2o is worth billions globally.
- Animal Feed: Used as vital preservatives in agriculture.
- De-icing: Utilized as eco-friendly de-icers for airport runways.
Investing in this technology is a smart business move. As regulations ban toxic chemicals, the demand for these safe, efficient alternatives is skyrocketing.
Frequently Asked Questions (FAQs)
What exactly is the hcooch ch2 h2o reaction?
It is the chemical process where methyl formate reacts with water (hydrolysis) to produce formic acid and methanol. It serves as a key industrial method for making clean, sustainable chemicals.
Is methyl formate dangerous to handle?
Yes, it is extremely flammable and can cause irritation to the eyes and respiratory system. It should only be handled in a controlled environment equipped with proper safety gear like gloves and ventilation systems.
Why is this reaction important for technology?
It produces high-purity formic acid, which is essential for manufacturing electronics, processing high-quality leather, and potentially storing hydrogen fuel for next-generation vehicles.
Can this reaction happen at room temperature?
It can occur, but it is relatively slow. In industrial settings, heat and pressure are applied to significantly speed up the hcooch ch2 h2o interaction and ensure efficiency.
What does the “CH2” stand for in the keyword?
In organic chemistry shorthand, CH2 usually refers to a methylene group. In this context, it is likely part of the structure of the ester (methyl group) or an intermediate step in the user’s search query regarding the molecule’s carbon chain.
Is this process environmentally friendly?
Yes, compared to older methods that produced significant salt waste, this hydrolysis process is very clean. The byproducts can be recycled, making it a gold standard in “green chemistry.”
Where can I buy chemicals for this reaction?
These are industrial-grade chemicals available from scientific suppliers. They are restricted substances and are usually sold only to registered businesses, research institutions, or universities.
Conclusion
The chemical system identified by hcooch ch2 h2o is more than just a string of letters; it is a cornerstone of modern chemical engineering. From preserving the food supply to potentially powering the electric vehicles of the future, the hydrolysis of methyl formate proves that simple molecules can have a massive impact.
By understanding the tech, safety protocols, and science behind this reaction, you gain insight into the invisible processes that keep our modern world running. Whether you are studying for a degree or are simply curious about science, remember that every great innovation starts with a simple reaction.